Solid State Fundamentals Part 2 – Conduction Electrons and Holes
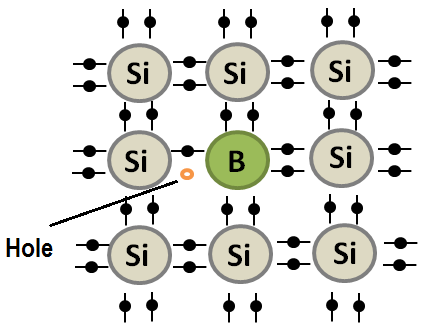
Conduction electrons and holes are fundamental concepts in solid state physics and are critical to understanding the behavior of semiconductors and the operation of semiconductor devices.
Conduction electrons are electrons that are free to move within the crystal lattice of a material and contribute to the material’s electrical conductivity. In semiconductors, conduction electrons come from the outermost energy band, also known as the conduction band.
In contrast, holes are the absence of an electron in a crystal lattice, and they behave like positive charges. Holes can be created in semiconductors through the process of doping, which involves adding impurities to the material. When an impurity atom is introduced to a semiconductor, it either donates or accepts an electron, creating either an excess of electrons or an absence of electrons in the crystal lattice.
Intrinsic semiconductors, which are pure semiconductors, have equal numbers of conduction electrons and holes. In this case, the conductivity of the material is very low. However, when impurities are added to the material through doping, the number of free electrons or holes can be increased, resulting in an increase in the conductivity of the material.
The interaction between conduction electrons and holes is the basis of many semiconductor devices, such as diodes and transistors.
The Band Gap
The band gap is a term used in solid-state physics and materials science to describe the energy difference between the valence band, which is the highest band of electrons that are bound to an atom in a material, and the conduction band, which is the lowest band of free electrons in a material.
In a solid material, the electrons are confined to a band structure, which consists of energy bands separated by band gaps. The valence band is the band that is occupied by the electrons in a material at zero temperature, and it is responsible for the material’s chemical properties. The conduction band, on the other hand, is the band above the valence band that contains free electrons, and it is responsible for the material’s electrical properties.
The band gap is the energy difference between the valence band and the conduction band. This energy gap determines whether a material is a conductor, an insulator, or a semiconductor. If the band gap is small, the material is a conductor because the electrons can easily jump from the valence band to the conduction band. If the band gap is large, the material is an insulator because the electrons require a large amount of energy to jump from the valence band to the conduction band.
In a semiconductor, the band gap is typically small enough that electrons can be excited from the valence band to the conduction band by thermal energy or by the application of a voltage. This makes semiconductors useful for electronic devices, where the band gap can be engineered to have a specific value to achieve desired electrical and optical properties.
Hole current vs Electron current
Hole current and electron current are two components of the total current flow in a semiconductor device.
Hole current refers to the flow of positive charge carriers (i.e., holes) through a semiconductor material. Holes are generated when an impurity atom (such as boron) is added to the semiconductor material, creating a p-type region with a deficit of electrons. In a p-type semiconductor, the holes act as the majority carriers, and they move through the material in response to an applied voltage, resulting in a hole current.
Electron current, on the other hand, refers to the flow of negative charge carriers (i.e., electrons) through a semiconductor material. Electrons are generated when an impurity atom (such as arsenic) is added to the semiconductor material, creating an n-type region with an excess of electrons. In an n-type semiconductor, the electrons act as the majority carriers, and they move through the material in response to an applied voltage, resulting in an electron current.
These videos will give you further insight.
Questions
References
- Band Gap and Semiconductor Current Carriers [Video]. (2016, August 22). Learn Engineering. https://youtu.be/N8MuD_xu6L4
- Basics of Semiconductors: Part 1 – What Makes a Semiconductor a Semiconductor? [Video]. (2015, September 3). EEVblog. https://youtu.be/5zz6LlDVRl0
- Kittel, C. (2004). Introduction to solid state physics. John Wiley & Sons.
- Ashcroft, N. W., & Mermin, N. D. (1976). Solid state physics. Saunders College.
- Sze, S. M., & Ng, K. K. (2006). Physics of semiconductor devices (3rd ed.). John Wiley & Sons.
- Ibach, H., & Lüth, H. (2009). Solid-state physics: An Introduction to principles of materials science (3rd ed.). Springer.

